Work Packages and deliverables
Work Package 1
Validation of tests for identified needs and specific pestsWP Leaders : Dr. Marcel Westenberg and Dr Maja Ravnikar (until M13)
WP Leaders : Dr Maja Ravnikar and Dr Géraldine ANTHOINE (from M14)
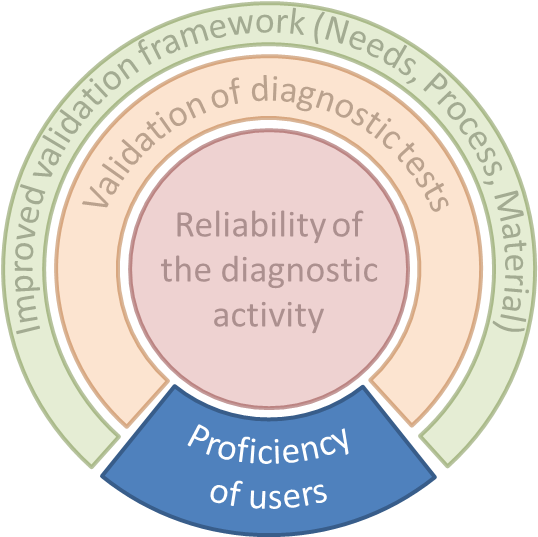
WP1 focus on validation of tests using different methods (i.e. (real-time) PCR, LAMP, ELISA, LFD) according to EPPO Standard PM 7/098, but improved especially by including a statistical approach developed in the frame work of WP2.
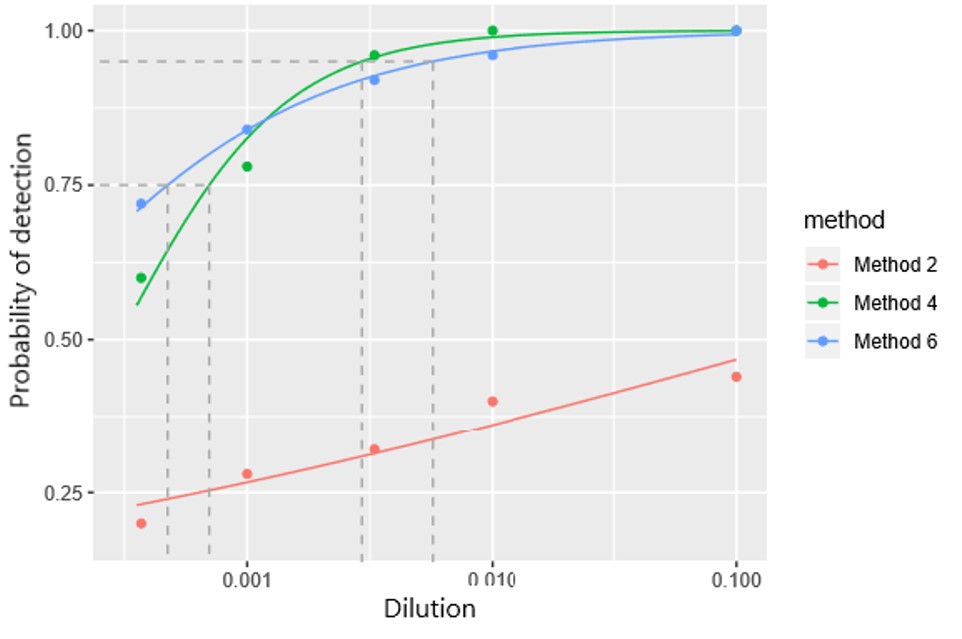
Performance criteria of several tests will be determined by organizing interlaboratory test performance studies (TPS) according to EPPO Standard PM 7/122 in which participating laboratories will use the same test for analysing identical sets of reference samples simulating the samples routinely analysed.
Two rounds of tests performance studies will be organised.
- The first round will be organized in early 2019 for six prioritized pests (Erwinia amylovora, Pantoea stewartii, citrus tristeza virus, plum pox virus, Fusarium circinatum and Bursaphelenchus xylophilus) in a range of matrices and for a range of methods.
- The second round will be performed in 2020 on pests and associated tests based on the needs of stakeholders and to the market analysed in the framework of WP4.

List of deliverables for WP1
- 1.1. Report detailing the minimum performance parameters to select tests for validation and selection of laboratories for TPS Download deliverable
- 1.2. List of tests for validation - Round 1 Download deliverable
- 1.3. List of tests for validation - Round 2 Download deliverable
- 1.4. TPS reports with description of the method, materials and software used, as well as the data analysis - Round 1
Download deliverable - 1.5. TPS reports with description of the method, materials and software used, as well as the data analysis - Round 2 Download deliverable
Once the deliverables will be finalised, they will be available here for download.
Work Package 2
Improvement of the validation processWP Leader : Dr Sebastien Massart
This WP aims to improve validation approaches for diagnostic technologies to maximise their usefulness for users (diagnosticians) and decision-makers (at National, European or Regional levels) and their use in routine diagnostics. In details, the WP aims at:
- Improving the current EPPO Standards for validation of tests for plant pest diagnostics (PM 7/098) and for the performance on interlaboratory comparisons (PM 7/122) by incorporating new statistical tools and predictive model;
- Developing best practice guidelines;
- Improving generic approaches for the validation and developing best practice guidelines for the validation and application of non-targeted (generic) diagnostic procedures, using high throughput sequencing (HTS or NGS) technologies for viruses detection, in plant pest diagnostics as a model.
List of deliverables for WP2
- 2.1. Guidelines for the revision of the EPPO Standards PM 7/098 and PM 7/122 for validation studies (including TPS) Download deliverable
- 2.2. “Best practice” guidelines for validation and routine use of non-targeted techniques in diagnostic setting which could serve as a basis for a new EPPO Standard Download deliverable
- 2.3. Scientific publication of the results of the validation of approach applied on 5 existing datasets Download deliverable
Once the deliverables will be finalised, they will be available here for download.
Work Package 3
Quality assurance for reference materials for validation purposesWP Leader : Prof. Dr René van der Vlugt
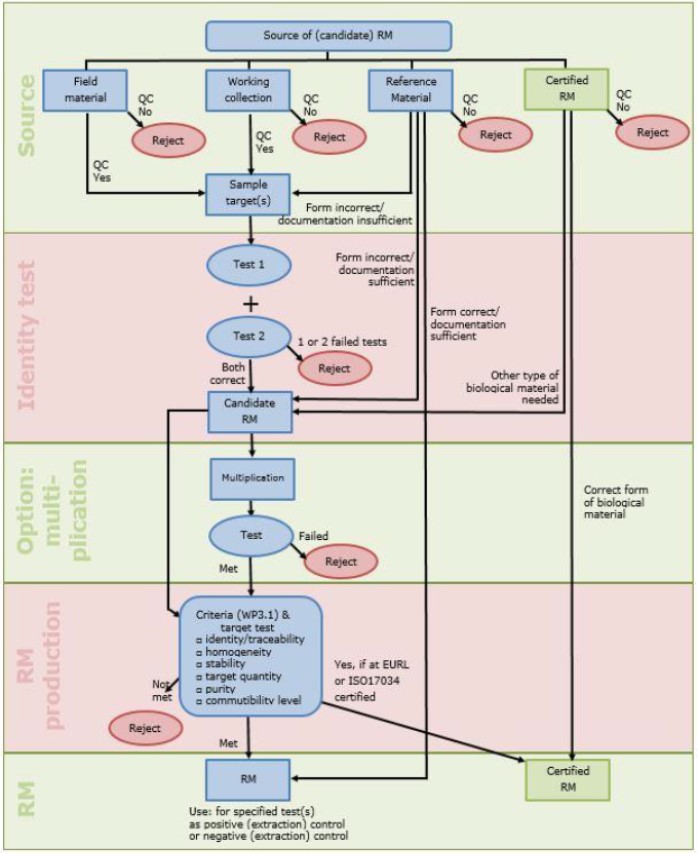
WP3 aims at developing recommendations for the quality of reference materials for validation purposes.
By building on existing guidelines provided by Q-collect and EPPO as well on the databases and collections present among the partners of the project’s consortium, WP3 will establish and disseminate (through WP6 and WP7) quality guidelines for the production and dissemination of reference materials for use in phytosanitary tests including possible quantification of targets in reference material for validation purpose. This will help in improving the overall standard of tests for plant pests in Europe and allow for a further implementation of shared standards and will introduce metrology principles in plant pest diagnostics.
Different types/classes of reference material (i.e. quantitative vs. qualitative) each have their own production characteristics thus require different guidelines. In consultancy with the end-users and building on the information already collected with Q-collect, these different needs and requirements will be defined and existing methods suitable for their characterization, including their commutability and the extent to which they need to resemble samples, will be evaluated and if necessary refined. The recommendations developed in this WP3 will be evaluated during the production of reference material in WP1.
In this way the guidelines will ensure that the reference materials produced shall match (as closely as possible) the plant materials encountered in routine testing both in terms of plant matrices as well as with respect to the infestation level of the specific pest.
List of deliverables for WP3
- 3.1. List of the criteria the reference materials have to meet for use in validation studies Download deliverable
- 3.2. Development draft Standard Operating Procedures (SOPs) available for the production of the reference materials identified in Task 3.1 Download deliverable
- 3.3. Guidelines and Standard Operating Procedures (SOP) finalised for the production of the reference materials Download deliverable
Once the deliverables will be finalised, they will be available here for download.
Work Package 4
Analysis of demand for testing and impactsWP Leader : Mr Glyn Jones
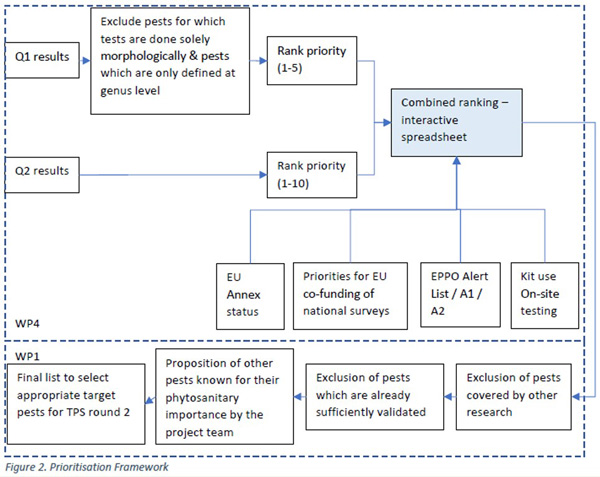
Work package 4 will assess the demand for current and future tests and operating procedures. Stakeholder feedback will be directly incorporated into the project through WP4, as it will form the basis for the prioritisation of tests in round two of the Test Performance Studies.
Through incorporating elements of the multi-actor approach and co-design with end-users, this approach will support plant health policies by ascertaining stakeholder views on attributes that lead to the adoption of surveillance and testing tools.
Furthermore, several market impact assessments will be conducted in close collaboration with WP7, to assess the scale of the effect of selected tests or toolkits in terms of monetised costs and benefits. A cost benefit approach (CBA) will be adopted, which will identify who benefits, when and at what scale; this information can then be used to assess the market potential for tests and toolkits.
List of deliverables for WP4
- 4.1. Report on stakeholder priorities for tests and general prioritization framework Download deliverable
- 4.2. Report on economic impact of priority tests Download deliverable
Once the deliverables will be finalised, they will be available here for download.
Work Package 5
Optimisation of proficiency evaluation for a horizontal assessmentWP Leader : Dr Mathieu Rolland
To ensure the proper use of a validated test in time, a monitoring of its use is required. The current approach is based on pest specific proficiency tests, it requires each laboratory to show its proficiency for each pest and test. Considering the difficulties of organising proficiency tests (time, cost, material availability, and complexity), all the pests and tests cannot be covered. This approach is no longer adequate and sustainable.
In VALITEST, experts will study the possibility to evaluate the proficiency of laboratories on tests (which have sufficient commonality in process and procedure) through a single PT. The needs of laboratories for PT will be collected and analysed in order to prepare guidelines on “horizontal” PT.
This action will provide guidelines which will be available for discussions with accreditation bodies.
List of deliverables for WP5
- 5.1. Analysis of the needs of the laboratories and applicability of the horizontal proficiency testing approach based on the questionnaire answers Download deliverable
- 5.2. Guidelines on an approach to undertake horizontal proficiency testing Download deliverable
Work Package 6
Dissemination, communication and trainingWP Leader : Ms Francoise Petter
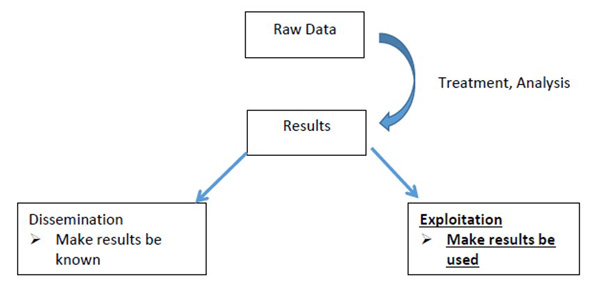
WP6 will be focused on communication, training and dissemination of the validation data and activities performed within the project. It will target the different identified stakeholder groups (including researchers, policy makers, inspectors, advisory services, diagnostic laboratories, industries) in order to facilitate the take-up and successful exploitation of the project results.
Training workshops on the guidance and frameworks developed in VALITEST will be carried out for diagnosticians in order to harmonise validation processes throughout the region and to ensure that they clearly understand and benefit from the project outputs. These Workshops will be organized in collaboration with WP7. Dissemination and training workshops will also be completed by the development of webinars and video tutorials on the validation process. Training for inspectors will also be included in the programme of EPPO Workshops for inspectors, and contact will be made with the EU Commission and coordinators of the programme Better Training for Safer Food (BTSF) to encourage synergies.
New validation data generated, or existing validation data gathered during the project will be made available through an improved version of the EPPO Database on diagnostic expertise (section validation data http://dc.eppo.int/validationlist.php ).
Finally, dissemination through scientific publications, technical articles and participations in appropriate events and congresses, will be prepared to present the different outcomes of the project.
List of deliverables for WP6
- 6.1. Project website and social media accounts Download deliverable
- 6.2. Survey on the needs of users of the EPPO Database and exploitation of the data Download deliverable
- 6.3. Inventory of validation data available Download deliverable
- 6.4. Dissemination and Training plan Download deliverable
- 6.5. Information materials (project fact-sheet, flyers, poster) Download deliverable
- 6.6. Report on improved version of the validation section of the EPPO Database on diagnostic expertise, including new data provided by laboratories Download deliverable
- 6.8. Report on the organisation of training activities (webinars, video tutorial, practical training sessions) (merge of original D6.7. Workshop for laboratories and D6.8. Webinar and video tutorials on the validation process) Download deliverable
- 6.9. Final Plan for the Dissemination and Exploitation of project Results (PDER) Download deliverable
Once the deliverables will be finalised, they will be available here for download.
Work Package 7
Market exploitation of the project resultsWP Leader : Mr Camilo Gianinazzi
WP7 will be dedicated to the promotion of the project’s results and market exploitation.
This WP, including all SME of the consortium, will promote the results of the project in connection with their commercial offer.
It will prepare the foundations for an EU Association of the Plant Health Diagnostics Industry and an EU Plant Health Diagnostics Charter for Industry. The drafting of these elements will be done while verifying that their application will be coherent with the SMEs’ business constraints.
Additionally, WP7 will contribute to dissemination workshops presenting use of kits validated in the framework of this project.
In association with WP4, WP7 will identify from the stakeholders needs, and the results of the projects for diagnostic tests that are not commercially available, the potential for developing new kits validated to consensus standards that could be swiftly brought to market. These new kits will be useful in field monitoring, eradication and containment campaigns for producers or competent authorities, and help to minimize economic and environmental damage as further explained in Section 2.
List of deliverables for WP7
- 7.1. Results exploitation plan Download deliverable
- 7.2. Communication and Exploitation strategy plans
- 7.3. Definition of the EU Plant Health Diagnostics Charter for Industry Download deliverable
- 7.4. Report on education and training session during the project Download deliverable
Once the deliverables will be finalised, they will be available here for download.
Work Package 8
Management of the consortium and the project.WP Leader : Dr Geraldine Anthoine
The objective of the project’s management is to guarantee the correct implementation of the activities, the achievement of the project objectives and expected results.
List of deliverables for WP8
- 8.1. Data Management Plan Download deliverable
- 8.2. Periodic report Download deliverable
- 8.3. Data Management Plan revised Download deliverable
- 8.4. Final report
- 8.5. Data Management Plan revised
Once the deliverables will be finalised, they will be available here for download.
Work Package 9
Ethics requirementsTo reach excellence, ethical compliance has to be considered as an integral part of research.
In VALITEST, it is fully integrated in the project; the dedicated WP9 will ensure that the project complies with fundamental ethical principles and legislation.
During the conception stage of the project, the protection of personnel data and the transfer of data and biological materiel to and from third countries have been identified.
A special attention will be paid to these two categories of potential ethical issues. Furthermore, during the ongoing project, the WP will monitor the research activity in order to detect potential new ethical issues and to ensure that the appropriate measures are taken.